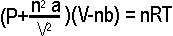

| P | Pressure in bar |
| V | Volume in Litres |
| n | Quantity of gas in mols |
| R | The universal gas constant, which is 0.0831451 if we want to do bar and litres |
| T | The temperature in Kelvins (virtually Centigrade+273) |
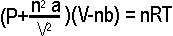
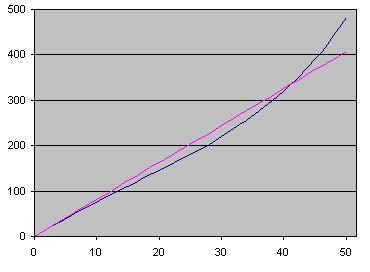
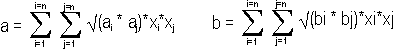
|
| |||
| Oxygen | 1.382 | 0.03186 | 31.9988 |
| Nitrogen | 1.370 | 0.03870 | 28.01348 |
| Helium | 0.0346 | 0.02380 | 4.0020602 |
| Air (treat as) | 1.3725 | 0.0372 | 28.85 |